NDSB 256(NDSB Reagents) SB256 Dimethylbenzylammonium-1-propane sulfonate[ 81239-45-4 ] f.w.: 257.4 daltons |
NDSB 256 is a non detergent sulfobetaine that prevents protein aggregation and facilitates the renaturation of chemically and thermally denatured proteins. Most enzymes remain active in the presence of these reagents. The applications are wide ranging in protein biochemistry useful to enhance extraction, solubilization and crystalization. The non detergent sulfobetaine reagents are zwitterionic, very soluble in water ( greater than 2.0 molar), do not alter significantly the pH or viscosity of biological buffers and can easily be removed by dialysis.
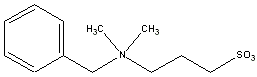
NDSB 256 is a non detergent sulfobetaine that prevents protein aggregation and facilitates the renaturation of chemically and thermally denatured proteins. Most enzymes remain active in the presence of these reagents. The applications are wide ranging in protein biochemistry useful to enhance extraction, solubilization and crystalization. The non detergent sulfobetaine reagents are zwitterionic, very soluble in water ( greater than 2.0 molar), do not alter significantly the pH or viscosity of biological buffers and can easily be removed by dialysis. NDSBs contain a hydrophilic sulfobetaine group and a short hydrophobic group. Hence, NDSBs cannot form micelles and are not considered detergents. Despite this, NDSBs have been used successfully to increase the yields of membrane, nuclear, and cytoskeletal associated proteins.It has been suggested that the NDSBs short hydrophobic group interacts with the hydrophobic regions on proteins to prevent aggregation. An analogy for using NDSB non detergent sulfobetaines with proteins and detergents would be to look at these reagents as fabric softeners. Examples: NDSB 256 will restore 30% of the enzymatic activity of denatured egg white lysozyme at 1 molar concentration; NDSB 256 will restore 16% of denatured beta-galactosidase at 800mM
- Vuillard, L., et al, Anal. Biochem, 230, 290 (1995)
- Vuillard, L., et al, J. Biochem., 305, 337 (1995)
- Vuillard, L., et al, FEBS Lett, 353, 294 (1994)
- Goldberg, M.E., et al, Folding and Design, 1, 21 (1996)
- FEBS Lett 1994 Oct 24;353(3):294-6
- Biochem J 1995 Jan 1;305 ( Pt 1):337-43
- Electrophoresis 1995 Mar;16(3):295-7
- Fold Des 1996;1(1):21-7
- Eur J Biochem 1998 Aug 15;256(1):128-35
- J.Cryst Growth (1996) vol 168 pp 150-154
- Anal. Biochem. (1995) 230, 290
- Prevents protein aggregation
- Facilitates the renaturation of chemically and thermally denaturated proteins
- Zwitterionic over a wide pH range
- Easily removed by dialysis



