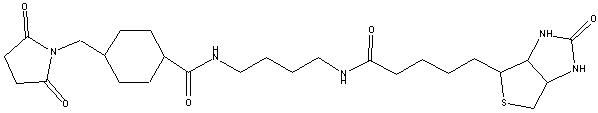
BIOTIN BMCC(Biotinylation Reagent) B111 (1-Biotinamido)-4[4-(maleimidomethyl)cyclohexanecarboxamido]hexanef.w.: 533.7 daltons |
Biotin BMCC is a maleimide activated, sulfhydryl reactive biotinylation reagent with an extended spacer arm that contains a stabilizing cyclohexane group.
| Quantity | Price | |
|---|---|---|
| 50mgm + | $247.00 |  |
| 100mgm + | $466.00 |  |
| Download***Certificate of Analysis*** | ||
Spacer arm 32.6 angstroms. Biotinylation reagent. Biotinylates at acidic or neutral pH. The reaction avoids potential modification of tyrosine groups that can inactivate biological activity. Soluble in DMSO and DMF.
- Atanasijevic, T., et al. (2006). Calcium-sensitive MRI contrast agents based on superparamagnetic iron oxide nanoparticles and calmodulin. Proc. Natl. Acad. Sci. USA 103:14707-12.
- Cadieux. N., et al. (2003). Differential substrate-induced signaling through the TonB-dependent transporter BtuB. Proc. Natl. Acad. Sci. USA 100:10688.
- Chenette, E.J., et al. (2006). Multiple sequence elements facilitate Chp rho GTPase subcellular location, membrane association, and transforming activity. Mol. Biol. Cell. 17:3108-21.
- Ching, K.H., et al. (2005). The role of SPECs, small Cdc42-binding proteins, in F-actin accumulation at the immunological synapse. J. Biol. Chem. 280:23660-7.
- Finkelstein, E.I., et al. (2005). Regulation of constitutive neutrophil apoptosis by the a,b-unsaturated aldehydes acrolein and 4-hydroxynonenal. Am. J. Physiol. Lung. Cell. Mol. Physiol. 289:L1019-28.
- Hou, H., et al. (2005). The DHHC protein Pfa3 affects vacuole-associated palmitoylation of the fusion factor Vac8. Proc. Natl. Acad. Sci. USA 102:17366-71.
- Luo, B-H., et al. (2004). Locking the b3 integrin I-like domain into high and low affinity conformations with disulfides. J. Biol. Chem. 279:10215-21.
- Politis, E.G., et al. (2005). Transmembrane topology of the protein palmitoyl transferase Akr1. J. Biol. Chem. 280: 10156-63.
- Wan, Y., et al. (2004). Increased site 1 affinity improves biopotency of porcine growth hormone: evidence against diffusion dependent receptor dimerization. J. Biol. Chem. 279:44775-84.
Protein labeling biotinylate antibodies or other proteins for use in protein methods
Membrane permeable Biotin BMCC can be used to label inside cells (intracellular)
Reacts with sulfhydryls (-SH), such as the side-chain of cysteine
Maleimide activated reactions at pH 6.5 to 7.5 in buffers such as PBS
Biotin BMCC is irreversible It forms permanent thioether bonds; spacer arm cannot be cleaved
Biotin BMCC must be dissolved in DMSO or DMF before further dilution in aqueous buffers
Medium length spacer arm (total length added to target) is 32.6 angstroms; contains cyclohexane ring, which stabilizes adjacent maleimide


