SMCC
(Protein Crosslinker heterobifunctional)
CL206
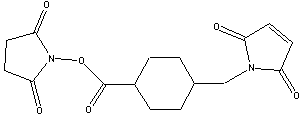
Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate
CAS#: [ 64987-85-5 ]
Formula Weight: f.w.: 334.3 daltons
SMCC is an amine to sulfhydryl crosslinker that contains NHS ester and maleimide reactive groups at opposite ends of a medium length cyclohexane stabilized spacer arm (8.3 angstroms).
Pricing & Ordering
| Quantity | Price | Action |
|---|---|---|
| 50mgm + | $90.00 | |
| 100mgm + | $170.00 |
Product Documents
- Ideal reagent for enzyme labeling of antibodies, both enzyme activity and antibody specificity can be preserved
- Create specific bioconjugates via one- or two-step crosslinking reactions
- Create sulfhydryl-reactive, maleimide-activated carrier proteins for coupling haptens
- Uto, I., et al. (1991). J. Immunol. Methods 138, 87-94.
- Bieniarz, C., et al. (1996). Extended Length Heterobifunctional Coupling Agents for Protein Conjugations. Bioconjug. Chem. 7, 88-95.
- Chrisey, L.A., et al. (1996). Nucleic Acids Res. 24(15), 3031-3039.
- Kuijpers, W.H., et al. (1993). Bioconjug. Chem. 4(1), 94-102.
- Brinkley, M.A. (1992). A survey of methods for preparing protein conjugates with dyes, haptens and crosslinking reagents. Bioconjugate Chem. 3, 2-13.
- Hashida, S., et al. (1984). More useful maleimide compounds for the conjugation of Fab to horseradish peroxidase through thiol groups in the hinge. J. Appl. Biochem. 6, 56-63.
- Mattson, G., et al. (1993). A practical approach to crosslinking. Molecular Biology Reports 17, 167-183.
- Partis, M.D., et al. (1983). Crosslinking of proteins by omega-maleimido alkanoyl N-hydroxysuccinimide esters. J. Protein. Chem. 2, 263-277.
- Samoszuk, M.K., et al. (1989). A peroxide-generating immunoconjugate directed to eosinophil peroxidase is cytotoxic to Hodgkins disease cells in vitro. Antibody, Immunoconjugates and Radiopharmaceuticals 2, 37-45.
- Yoshitake, S., et al. (1982). Mild and efficient conjugation of rabbit Fab and horseradish peroxidase using a maleimide compound and its use for enzyme immunoassay. J. Biochem. 92, 1413-1424.
- Amine reactive NHS ester crosslinks rapidly with primary amine-containing molecule
- Sulfhydryl-reactive maleimide reacts with cysteine residues to yield specific conjugates
- High purity, crystalline SMCC can be used to create high-purity maleimide-activated derivatives
- Cyclohexane bridge confers added stability to the maleimide group making SMCC an ideal crosslinking agent for maleimide activation of proteins. Maleimide groups are stable for 64 hours in 0.1 M sodium phosphate buffer, pH 7 at 4°C.


